Subtitle
A Pilot Trial of Proton Based Cardiac Sparing Accelerated Fractionated RadioTherapy in Unresectable Non-Small Cell Lung Cancer with Extended Durvalumab Therapy (PARTICLE-D).
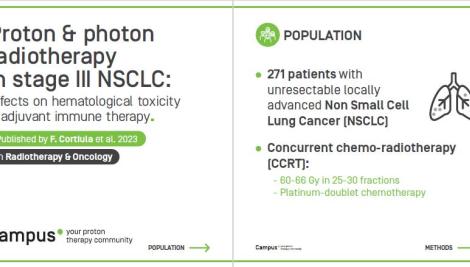
This phase I dose-escalation study by the group of Cleveland Medical Center, Ohio, reported severe treatment related toxicity. A total of 7 patients with stage IIIA-IIIC NSCLC were enrolled in this study, including 5 patients to arm one at total of 60 Gy in 20 fractions, and 2 patients in arm two at total of 69 Gy in 23 fractions. After a median follow-up of 15 months, the overall response rate was 71.4% with 5 partial response and 2 with stable disease. The median PFS and OS were 15 months and 16.4 months respectively. This study reported three treatment related death, including one grade 3 pneumonitis occurred in arm one within 30 days of proton-RT completion which further developed to grade 5 pneumonia; two late-onset grade 5 tracheal necroses occurred 415 and 413 days following completion of proton-RT – one patient each in arms one and two. In view of the three treatment related fatalities, the authors stated that it was deemed unsafe to continue to accruing patients to the trial. In discussion about the treatment related fatalities, the authors remarked that it was possible that the combination of tumor location (i.e. central disease, close to airway), hypofractionation and passive scatter technique contributed to this outcome.